

:max_bytes(150000):strip_icc()/precipitate-589cb8953df78c47581a9014.jpg)
The measure of an element’s ability to lose electrons is known as electro-positivity, and it increases as we move down a column.
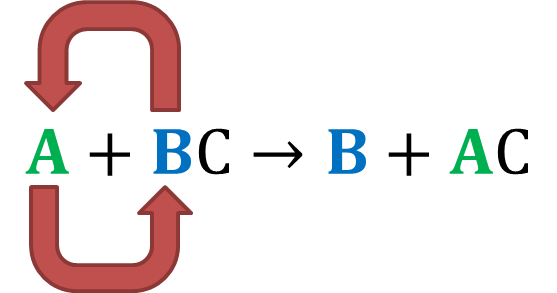
They illustrate the poorest nuclear attraction and consequently have a poor hold on their outermost electrons. Heavy metals have an increased radius, especially those on the utmost bottom. This is because the increase in electrons is paralleled by an increase in shells. The most electro-negative element is the likeliest to steal electrons and react the quickest.Īnother trend is the increase in the radius of elements as we move down a column, while the number of valence electrons remains the same, even though the atomic number keeps increasing. The measure of an element’s ability to pull electrons towards it is known as electronegativity. Therefore, due to a strong pull of attraction, an element is more likely to buy or gain electrons as we move along a row. In the tug-o’-war between the pulling protons and the incrementing electrons, the former wins, resulting in a greater nuclear attraction, pulling all its shells closer to it. The reason being, the increase in electrons is being mirrored by the increase in protons in the center, whereas the former must cram into the same shell. For instance, every element in the second row will contain only two shells and so on.Ī consequence of this trend is that atomic radius decreases as we move towards a row’s last resident. The number of shells can be deduced from the row number. The number of electrons in the valence shell increases by one as we parse through every element in a row, although the number of shells does remain the same. (Isn’t it weird that they tend to be exactly the same?) Trend 1 The table arranges elements according to their atomic number, which is same as the number of protons or electrons they encompass. The periodic table organizes every element we know of in a collocated manner. The periodic table is a reminder that scientists are borderline OCD and cannot help but organize everything in an extremely neat and ordered way. How do we find out whether an element is a buyer or seller? We refer to the periodic table. How do we estimate an element’s incentives? The periodic table and its “trends” Thus, reactivity is a function of how easily an element loses or gains electrons. Buying six when you can simply lose two would be outright foolish, but more importantly, expensive! This makes sense, as an element would prefer to lose electrons and render itself empty if its outermost shell is less than half-filled, or gain some to fill itself, if it is more than half-filled. Anyone who’s familiar with nature’s parsimonious way of functioning will guess that trades requiring the least amount of energy are the ones that are most likely to occur. The fewer the electrons involved, the lesser the energy expense. Basically, an element can buy more electrons to fill its penultimate shell or sell them to empty it. The purpose of this trade is to attain stability, an optimal configuration of electrons, which is achieved when either an element’s valence (outermost) shell is completely filled or emptied. The goods are electrons and the currency being exchanged is energy.
/close-up-of-a-burning-matchstick-574900877-58b5b3575f9b586046bc5fae.jpg)
The buyers and sellers are chemical elements. In simple terms, a chemical reaction is the perfect opportunity for buyers and sellers to buy and sell goods. What determines reactivity of an element? So, the question becomes – which element is the easiest to incite? But first… Reactivity, on the other hand, can be defined as the measure of how readily a chemical species will participate in a reaction and form chemical bonds. This is exactly what we’re concerned with right now.ĭevoid of any technical jargon, a “reaction” is exactly what it means, a response. Every now and then, somebody mumbles “reaction” or “reactivity”, terms so ubiquitous that you’ll find them infiltrating chemistry books to their very last page. What did one chemist say to another when he found him aggrieved? “Why are you sodium distressed? I think you’re over-reacting!” See what I did there? Sometimes I wonder why – despite my hilarity – I find it really hard to make friends…Īnyway, other than my poor grades, the first thing that comes to mind when I think of chemistry is lab coats, oddly shaped vessels, as if reflections from carnival mirrors, and blazing explosions. Caesium is the most reactive metal in the periodic table, so much that working with this metal often ends in explosions! How do we estimate an element’s incentives?įluorine is identified as the most reactive nonmetal and the most electronegative element in the periodic table, making it the strongest oxidizing agent.What determines reactivity of an element?.


 0 kommentar(er)
0 kommentar(er)
